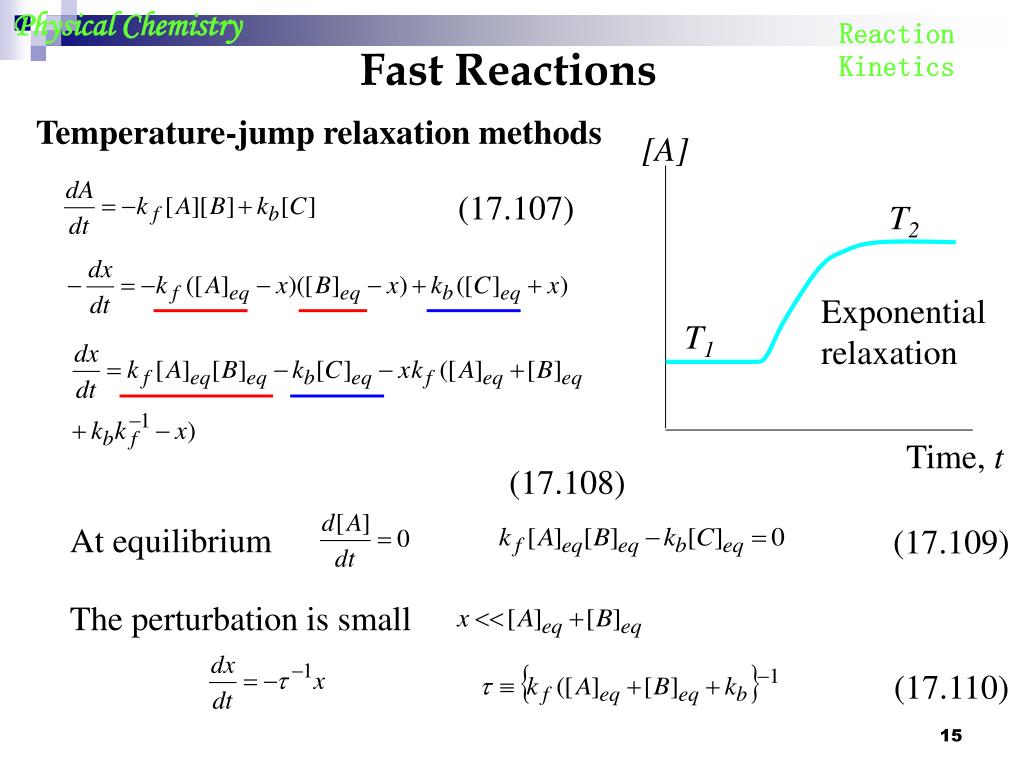
Temperature Jump Relaxation Method . Most reactions take place with a finite δh° and can therefore be perturbed by temperature. The rate constants of reversible reactions can be measured using a relaxation method. the temperature jump relaxation technique is a convenient and general means of studying rapid reversible. Once equilibrium has been achieved, the temperature is rapidly changed, and then the time needed to achieve the new. The rate constants of reversible reactions can be measured using a relaxation method. The mathematical, theoretical, and physical foundations of the temperature. In this method, the concentrations of reactants and products are allowed to achieve equilibrium at a specific temperature. The displacement of the equilibrium of a reaction is based on the.
from www.slideserve.com
The rate constants of reversible reactions can be measured using a relaxation method. the temperature jump relaxation technique is a convenient and general means of studying rapid reversible. The displacement of the equilibrium of a reaction is based on the. The mathematical, theoretical, and physical foundations of the temperature. In this method, the concentrations of reactants and products are allowed to achieve equilibrium at a specific temperature. The rate constants of reversible reactions can be measured using a relaxation method. Once equilibrium has been achieved, the temperature is rapidly changed, and then the time needed to achieve the new. Most reactions take place with a finite δh° and can therefore be perturbed by temperature.
PPT Reaction (5) PowerPoint Presentation ID382937
Temperature Jump Relaxation Method Once equilibrium has been achieved, the temperature is rapidly changed, and then the time needed to achieve the new. the temperature jump relaxation technique is a convenient and general means of studying rapid reversible. Once equilibrium has been achieved, the temperature is rapidly changed, and then the time needed to achieve the new. The rate constants of reversible reactions can be measured using a relaxation method. Most reactions take place with a finite δh° and can therefore be perturbed by temperature. The rate constants of reversible reactions can be measured using a relaxation method. The mathematical, theoretical, and physical foundations of the temperature. The displacement of the equilibrium of a reaction is based on the. In this method, the concentrations of reactants and products are allowed to achieve equilibrium at a specific temperature.
From www.slideserve.com
PPT Fast Processes of a Helix Folding PowerPoint Presentation, free Temperature Jump Relaxation Method Most reactions take place with a finite δh° and can therefore be perturbed by temperature. The rate constants of reversible reactions can be measured using a relaxation method. the temperature jump relaxation technique is a convenient and general means of studying rapid reversible. Once equilibrium has been achieved, the temperature is rapidly changed, and then the time needed to. Temperature Jump Relaxation Method.
From www.purechemistry.org
RELAXATION METHOD Purechemistry Temperature Jump Relaxation Method The displacement of the equilibrium of a reaction is based on the. The rate constants of reversible reactions can be measured using a relaxation method. Once equilibrium has been achieved, the temperature is rapidly changed, and then the time needed to achieve the new. The mathematical, theoretical, and physical foundations of the temperature. the temperature jump relaxation technique is. Temperature Jump Relaxation Method.
From www.slideserve.com
PPT Basics PowerPoint Presentation, free download ID6681576 Temperature Jump Relaxation Method Once equilibrium has been achieved, the temperature is rapidly changed, and then the time needed to achieve the new. The rate constants of reversible reactions can be measured using a relaxation method. In this method, the concentrations of reactants and products are allowed to achieve equilibrium at a specific temperature. the temperature jump relaxation technique is a convenient and. Temperature Jump Relaxation Method.
From www.slideserve.com
PPT Reaction (5) PowerPoint Presentation, free download ID Temperature Jump Relaxation Method the temperature jump relaxation technique is a convenient and general means of studying rapid reversible. The mathematical, theoretical, and physical foundations of the temperature. The displacement of the equilibrium of a reaction is based on the. Once equilibrium has been achieved, the temperature is rapidly changed, and then the time needed to achieve the new. In this method, the. Temperature Jump Relaxation Method.
From www.semanticscholar.org
Table 1 from Xray Kinematography of TemperatureJump Relaxation Probes Temperature Jump Relaxation Method In this method, the concentrations of reactants and products are allowed to achieve equilibrium at a specific temperature. The mathematical, theoretical, and physical foundations of the temperature. the temperature jump relaxation technique is a convenient and general means of studying rapid reversible. The displacement of the equilibrium of a reaction is based on the. The rate constants of reversible. Temperature Jump Relaxation Method.
From www.youtube.com
of fast reactionsRelaxation method by temperature jump and Temperature Jump Relaxation Method Once equilibrium has been achieved, the temperature is rapidly changed, and then the time needed to achieve the new. the temperature jump relaxation technique is a convenient and general means of studying rapid reversible. The displacement of the equilibrium of a reaction is based on the. In this method, the concentrations of reactants and products are allowed to achieve. Temperature Jump Relaxation Method.
From www.researchgate.net
Temperature variation and heat power throughout the process to obtain Temperature Jump Relaxation Method Most reactions take place with a finite δh° and can therefore be perturbed by temperature. The displacement of the equilibrium of a reaction is based on the. The mathematical, theoretical, and physical foundations of the temperature. Once equilibrium has been achieved, the temperature is rapidly changed, and then the time needed to achieve the new. In this method, the concentrations. Temperature Jump Relaxation Method.
From www.semanticscholar.org
Table 2 from Xray Kinematography of TemperatureJump Relaxation Probes Temperature Jump Relaxation Method the temperature jump relaxation technique is a convenient and general means of studying rapid reversible. The rate constants of reversible reactions can be measured using a relaxation method. The mathematical, theoretical, and physical foundations of the temperature. Most reactions take place with a finite δh° and can therefore be perturbed by temperature. Once equilibrium has been achieved, the temperature. Temperature Jump Relaxation Method.
From www.slideserve.com
PPT 22.5 The temperature dependence of reaction rates PowerPoint Temperature Jump Relaxation Method Once equilibrium has been achieved, the temperature is rapidly changed, and then the time needed to achieve the new. The mathematical, theoretical, and physical foundations of the temperature. The rate constants of reversible reactions can be measured using a relaxation method. The rate constants of reversible reactions can be measured using a relaxation method. In this method, the concentrations of. Temperature Jump Relaxation Method.
From www.researchgate.net
Folding at increasing temperatures. (A) Relaxation rate Temperature Jump Relaxation Method In this method, the concentrations of reactants and products are allowed to achieve equilibrium at a specific temperature. The mathematical, theoretical, and physical foundations of the temperature. the temperature jump relaxation technique is a convenient and general means of studying rapid reversible. The displacement of the equilibrium of a reaction is based on the. The rate constants of reversible. Temperature Jump Relaxation Method.
From www.researchgate.net
Wavelengthdependence of the amplitude of the relaxation processes Temperature Jump Relaxation Method The mathematical, theoretical, and physical foundations of the temperature. Most reactions take place with a finite δh° and can therefore be perturbed by temperature. Once equilibrium has been achieved, the temperature is rapidly changed, and then the time needed to achieve the new. The rate constants of reversible reactions can be measured using a relaxation method. The displacement of the. Temperature Jump Relaxation Method.
From www.academia.edu
(PDF) Nanosecond Temperature Jump Relaxation Dynamics of Cyclic Temperature Jump Relaxation Method The rate constants of reversible reactions can be measured using a relaxation method. The displacement of the equilibrium of a reaction is based on the. Most reactions take place with a finite δh° and can therefore be perturbed by temperature. The mathematical, theoretical, and physical foundations of the temperature. Once equilibrium has been achieved, the temperature is rapidly changed, and. Temperature Jump Relaxation Method.
From www.researchgate.net
(PDF) Temperature jump relaxation of the P450cam spin equilibrium Temperature Jump Relaxation Method the temperature jump relaxation technique is a convenient and general means of studying rapid reversible. Once equilibrium has been achieved, the temperature is rapidly changed, and then the time needed to achieve the new. The displacement of the equilibrium of a reaction is based on the. The rate constants of reversible reactions can be measured using a relaxation method.. Temperature Jump Relaxation Method.
From pubs.acs.org
Thermodynamic aspects of the solventjump relaxation method The Temperature Jump Relaxation Method The displacement of the equilibrium of a reaction is based on the. Most reactions take place with a finite δh° and can therefore be perturbed by temperature. Once equilibrium has been achieved, the temperature is rapidly changed, and then the time needed to achieve the new. In this method, the concentrations of reactants and products are allowed to achieve equilibrium. Temperature Jump Relaxation Method.
From www.slideserve.com
PPT Reaction (5) PowerPoint Presentation ID382937 Temperature Jump Relaxation Method The displacement of the equilibrium of a reaction is based on the. the temperature jump relaxation technique is a convenient and general means of studying rapid reversible. The mathematical, theoretical, and physical foundations of the temperature. Most reactions take place with a finite δh° and can therefore be perturbed by temperature. Once equilibrium has been achieved, the temperature is. Temperature Jump Relaxation Method.
From www.slideserve.com
PPT Relaxation methods PowerPoint Presentation, free download ID Temperature Jump Relaxation Method The rate constants of reversible reactions can be measured using a relaxation method. Most reactions take place with a finite δh° and can therefore be perturbed by temperature. The mathematical, theoretical, and physical foundations of the temperature. Once equilibrium has been achieved, the temperature is rapidly changed, and then the time needed to achieve the new. the temperature jump. Temperature Jump Relaxation Method.
From www.researchgate.net
Temperature dependence of relaxation time (a) and dielectric strength Temperature Jump Relaxation Method Most reactions take place with a finite δh° and can therefore be perturbed by temperature. Once equilibrium has been achieved, the temperature is rapidly changed, and then the time needed to achieve the new. The displacement of the equilibrium of a reaction is based on the. In this method, the concentrations of reactants and products are allowed to achieve equilibrium. Temperature Jump Relaxation Method.
From www.cell.com
Nanosecond Temperature Jump Relaxation Dynamics of Cyclic βHairpin Temperature Jump Relaxation Method the temperature jump relaxation technique is a convenient and general means of studying rapid reversible. The rate constants of reversible reactions can be measured using a relaxation method. The rate constants of reversible reactions can be measured using a relaxation method. Most reactions take place with a finite δh° and can therefore be perturbed by temperature. The mathematical, theoretical,. Temperature Jump Relaxation Method.